Learn About Allografts

Soft tissue grafts are used in several sports medicine indications, including, but not limited to, rotator cuff repair, ligament/tendon reinforcement, and nerve wrapping. Grafting options include autograft, sterile and non-sterile allograft, xenograft, and synthetic options.
Soft tissue allograft solutions are available for procedures ranging from ACL repairs to complex reconstructions. These can include tendons, dermis, cartilage, osteoarticular, and fresh allografts. This section focuses on soft tissue grafts used in ligament/tendon reconstruction and rotator cuff repair.
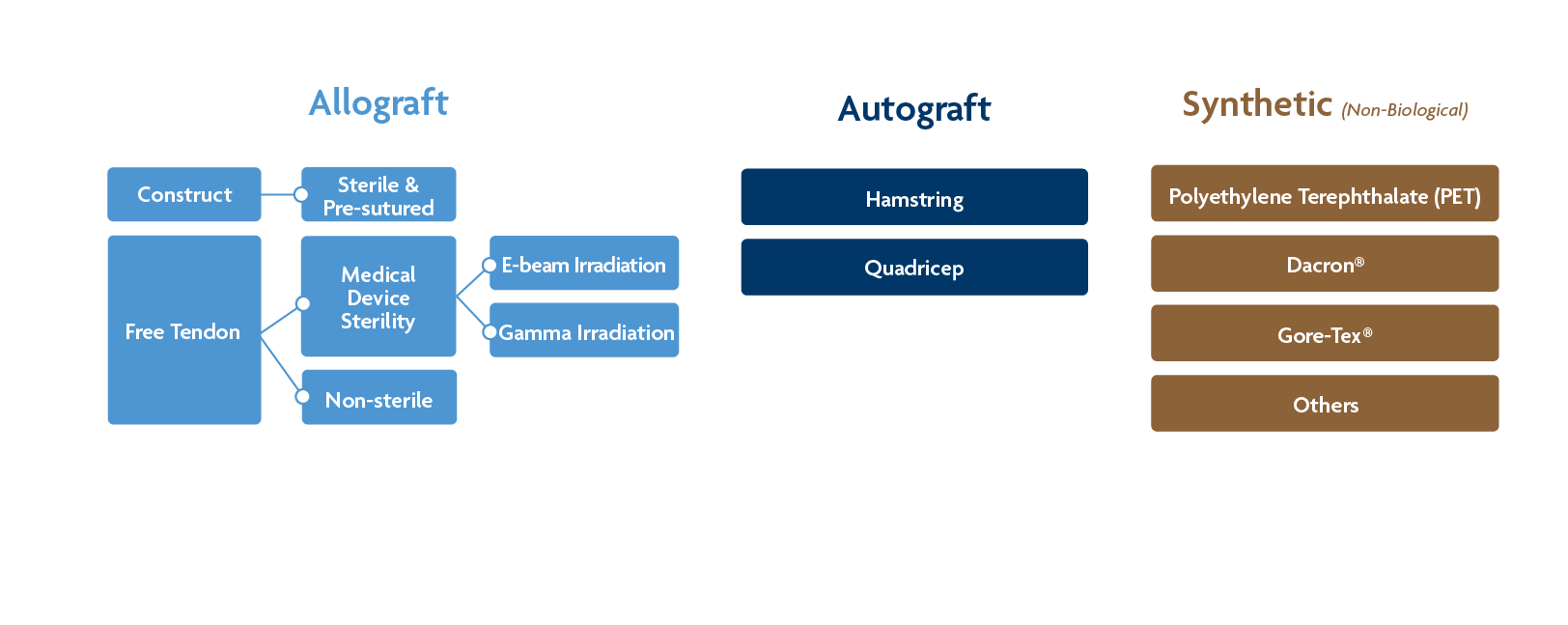
Autograft tendons – a whole or portion of a tendon taken from a second surgical site on the patient.
Allograft tendons – Tendon recovered from a deceased donor (with family authorization). May include attached bone blocks. May remain in its native form or be made into a construct with sutures. Cleansing and disinfection methods can vary widely been tissue processors.
Allograft tendon constructs – this option is pre-sutured and varies in the number of strands included. This type of graft is considered a medical device and is provided sterile.
Non-sterile allograft tendons – Some tissue processors only cleanse and disinfect tendon allografts via aseptic processing, which may result in the tissue being culture negative, but does result in a sterile graft. Note: while some processors do not use terminal sterilization, they may still employ gamma irradiation during their aseptic processing to reduce bioburden, but not terminally sterilize the tissue.1
Allograft tendons with medical device sterility – These tendons are provided at a Sterility Assurance Level (SAL) of 10-6, meaning that the probability of finding a single viable microorganism is one in one million.2
- Sterilization with e-beam irradiation - This type of sterilization uses beta particles to disrupt nucleic acids. The irradiation type has less penetration depth compared to gamma irradiation.4
- Sterilization with gamma irradiation – The most common method of terminal sterilization. The type of irradiation uses high energy photons to disrupt nucleic acids and penetrates deep into the tissue, which is particularly important for the safety of dense tissues.5
Synthetic tendons/ligaments – These tendon substitutes can be made from carbon, polyester, Dacron, and other textiles. These grafts have limited clinical data.6
Grafts for Shoulder Repair
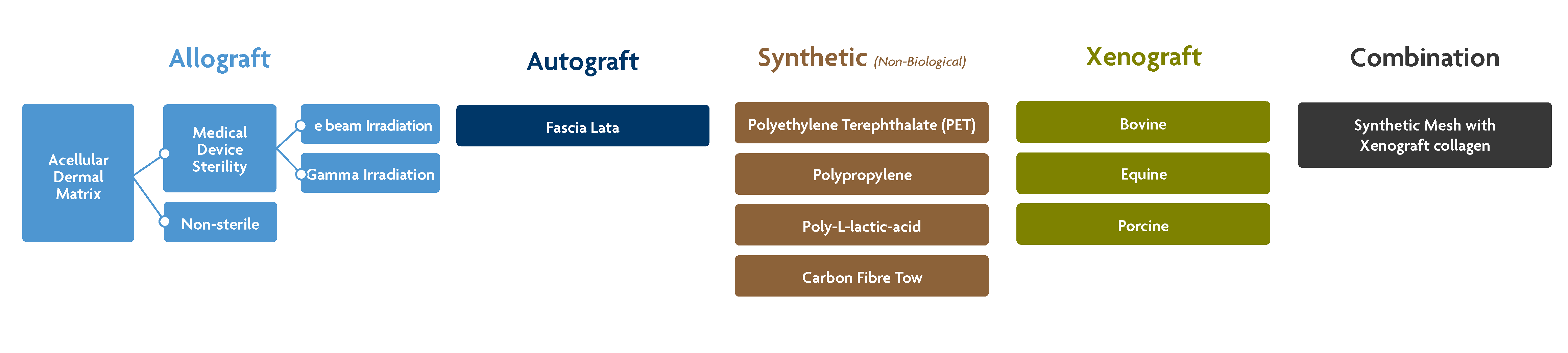
Autograft – A soft tissue graft taken locally or from a second surgery site. An example would be the use of autograft fascia lata for superior capsule reconstruction.
Acellular Dermal Matrix (ADM) Allograft – A thick skin graft recovered from a deceased donor (with family authorization). Decellularization, cleansing, and disinfection methods can vary widely been tissue processors.
- Non-sterile dermal allograft – Some tissue processors only cleanse allografts via aseptic processing, which leaves the graft negative for bacterial cultures, but does not ensure sterility. Note: while some processors do not use terminal sterilization, they may still employ gamma irradiation during their aseptic processing.1
- Allograft acellular dermal matrix with medical device-level sterility – These decellularized ADMs are provided at a sterility assurance level (SAL) of 10-6 , meaning that the probability of finding a single viable microorganism is one in one million.2
- Sterilization with e-beam irradiation – This type of sterilization uses beta particles to disrupt nucleic acids. The irradiation type has less penetration depth compared to gamma irradiation.4
- Sterilization with gamma irradiation – The most common method of terminal sterilization. The type of irradiation uses high energy photons to disrupt nucleic acids and penetrates deep into the tissue.5
Synthetic options for rotator cuff repair– These materials can be made from a variety of polymers.7
Xenograft options for rotator cuff repair– These grafts may be derived from cows, pigs, or horses.7 Processing varies widely between manufacturers.
References
- https://www.mtfbiologics.org/docs/default-source/packageinserts/pi-3_rev_23_2022.pdf Accessed on 6/29/2022
- Sterilization Unwrapped (infectioncontroltoday.com) Accessed 6/28/2022 at 2:54pm
- Farago, D., Kozma, B. & Kiss, R.M. Different sterilization and disinfection methods used for human tendons – a systematic review using mechanical properties to evaluate tendon allografts. BMC Musculoskelet Disord 22, 404(2021). https://doi.org/10.1186/s12891-021-04296-4
- https://www.steri-tek.com/ebeam/#:~:text=Ebeam%20irradiation%20is%20a%20proven%20and%20accepted%20form,and%20breaking%20up%20the%20RNA%2FDNA%20of%20the%20bioburden. Accessed 6/28/2022 at 2:30pm
- https://www.steris-ast.com/techtip/introduction-gamma-irradiation-processing/ Accessed 6/28/2022 at 2:30pm.
- Abdullah S. Usage of synthetic tendons in tendon reconstruction. BMC Proc. 2015 May 19;9(Suppl 3):A68. doi:10.1186/1753-6561-9-S3-A68. PMCID: PMC4444979.
- Karuppaiah, K., & Sinha, J. (2019). Scaffolds in the management of massive rotator cuff tears: current concepts and literature review, EFORT Open Reviews, 4(9), 557-566. Retrieved Jul 12, 2022, from https://eor.bioscientifica.com/view/journals/eor/4/9/2058-5241.4.180040.xml